Useful! Why Is Fractional Distillation More Effective Than Simple
The initial composition of the unknown was 461 cyclohexane and 539 toluene. The fractionating column is placed.
Free Ncert Solutions For 9th Class Science Is Matter Around Us Pure Studyadda Com
Fractional distillation is used to separate liquid mixtures where the compounds have similar boiling points andor they are present in comparable amounts.

Why is fractional distillation more effective than simple. The fractional distillation was more time consuming than the simple but provided a more concentrated product. Here is the excerpt of my lab manual. And possible multiple fractional distillations would have been needed to obtain a more complete separation.
Thankfully this is exactly why fractional distillation is so effective for cannabis and hemp oil extraction. As more ethanol collected in the receiving flask and water remained in distilling flask the overall distillation temperature would increase before leveling out. Then is fractional distillation more efficient than simple distillation.
Fractional Distillation Vs. This type of distillation is an equilibrium process in which the composition of the distillate is constantly changing as the distillation proceeds. If two liquids have a boiling point difference of less than 40-50 oC simple distillation will not be effective at separating them.
Fractional distillation is more efficient in separating ideal solutions into their pure components than simple distillation. Ethanol is more volatile than water because its vapour pressure is far higher around X5 higher. A fractional distillation is used when separating mixtures of liquids whose boiling points are similar separated by less than 70 o C.
Explain why a packed fractional distillation column is more efficient than an unpacked column for separating two closely boiling liquids. The fractional distillation is more efficient and is suited for mixtures of volatile liquidsThe closer the difference in the boiling points the more demanding the distillation. The time required for fractional distillation was greater than the time needed for simple distillation but it was a much more accurate distillation.
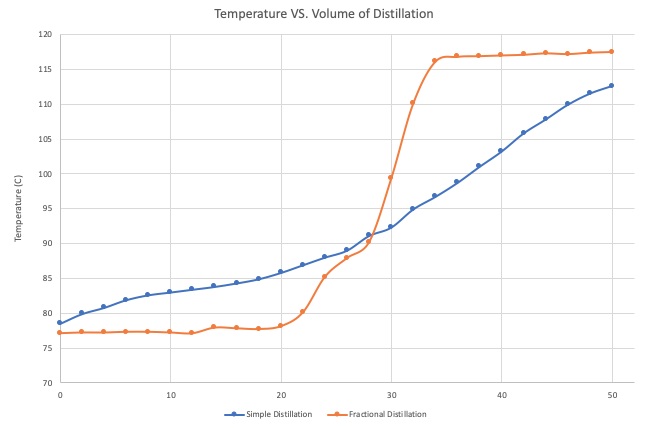
Fractional distillation is used when a more efficient separation process than simple distillation is required. A comparison of the head temperature versus distillate volume graphs in Figures 3 and 4 shows that fractional distillation is much more effective at isolating volatile organic liquids. Its possible to isolate chemicals using simple distillation but it requires careful control of the temperature because only one fraction can be isolated at a time.
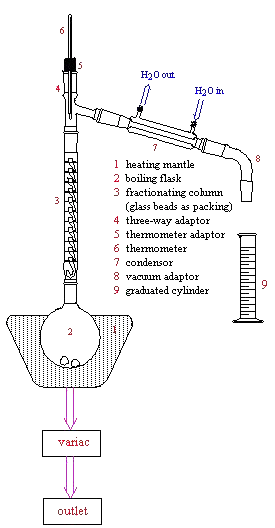
The main element of the apparatus is the distillation column which consists of a. Some evaporates into the air and some is left behind stuck to the apparatus. Fractional distillation leads to a better separation than simple distillation because the glass beads in the fractionating column provide theoretical plates on which the vapors can condense and then re-evaporate and re-condense essentially distilling the compound many times over.
Fractional distillation differs from simple distillation because the fractionating column naturally separates compounds based on their boiling points. Distillation is the standard process used for separation of chemical mixtures. Thus for this mixture three simple distillations have produced the desired purification.
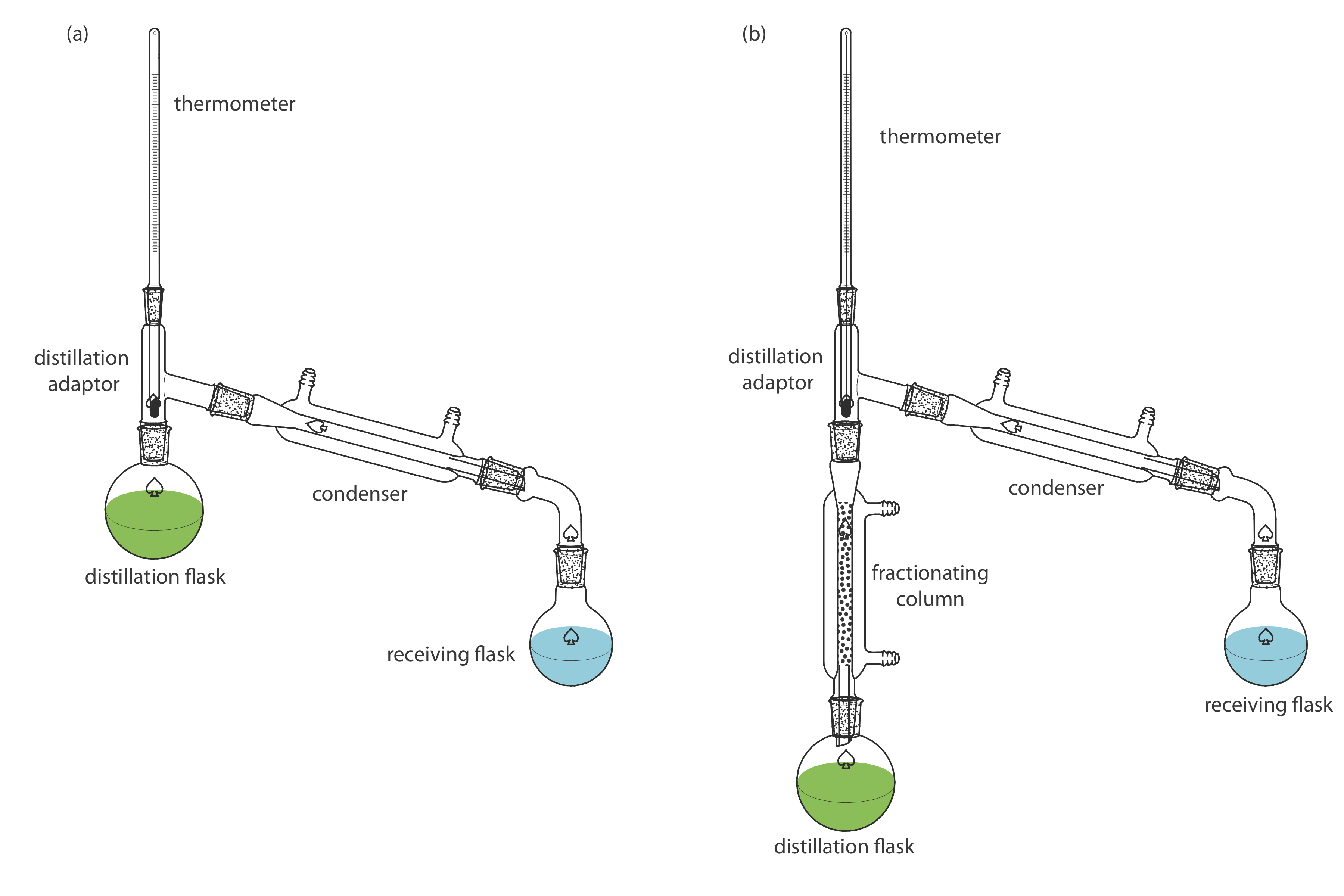
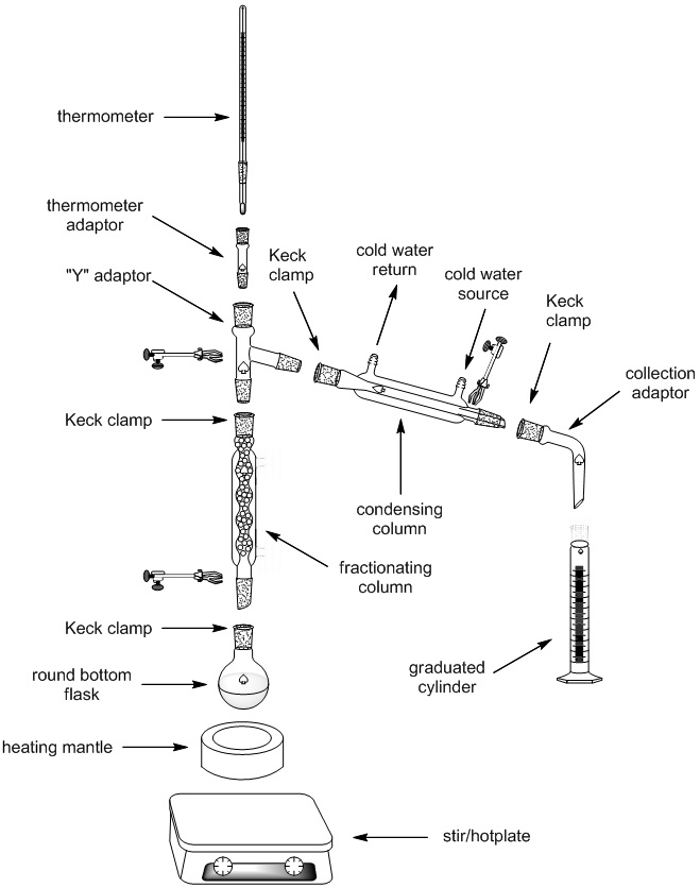
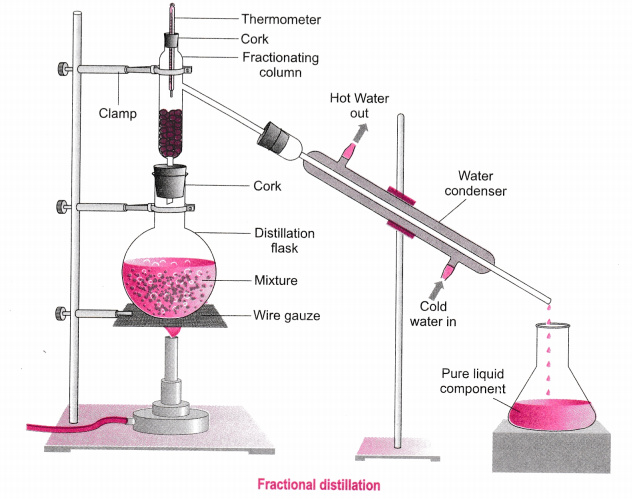
In this article a comparison between fractional and simple distillation has been presented which will identify the prime differences between the two processes. It includes distilling flask condenser receiver fractionating column thermometer and heat source. For solutions that deviate slightly from Raoults law the method can still be applied for complete separation.
The fractional distillation is more efficient and is suited for mixtures of volatile liquids. The difference between simple and fractional distillation is the number of times that the liquid is vaporized and condensed. Few fractional distillation apparatuses are required for the process.
A packed fractional distillation column will be more effective at separating two closely boiling liquids than an empty one because you have more surfaces for condensation and revaporization of. Unfortunately each time a distillation is run material is lost. Fractional distillation gives better separation between the liquids is because the glass beads in the fractionating column provide theoretical plates on which the refluxing liquid can condense re-evaporate and condense again essentially.
In this case fractional distillation must be used. Each vaporization-condensation step is known as a simple distillation. In a fractional distillation a mixture of liquids is boiled and the resulting vapors travel up a glass tube called a fractionating column and separate.
Answer 1 of 2. The sponge helped improve efficiency of the distillation. After setting up the apparatus a mixture of two miscible liquids A and B is taken where A has more volatility than substance B.
This can be seen on the graphs. When the distillation of ethyl alcohol is carried out it is a well known fact that water and ethanol form an azeotrope giving a combined boiling point lower than that of ethanol. Fractional distillation allows for several condensation-condensation cycles in a single operation.
What is the reason as to why Fractional Distillation is more effective than Simple Distillation. Fractional distillation is more efficient than simple distillation because it can perform several simple distillations in one apparatus. The drawback is that fractional distillations typically take longer because we want to achieve pseudo-equilibrium between vapor and liquid throughout this system.
Besides why fractional distillation is more efficient than simple distillation. Simple distillation is more useful for purifying a liquid that contains either a non-volatile impurity or small amounts of higher or lower boiling impurities. By using fractional distillation and other world-class extraction equipment your company will be able to create a finished product with over 99 percent purity.
The closer the difference in the boiling points the more demanding the distillation. For mixtures that contain only one volatile component a simple distillation can be more than sufficient. Experimental Organic Chemistry 6th Edition Edit edition Solutions for Chapter 44 Problem 4E.
Simple distillation condenses the liquid once so the boiling points of the two.
Simple And Fractional Distillation Mendelset
Solved Based Only On The Data On The Graph Explain Why The Chegg Com

Simple Distillation Vs Fractional Distillation Distillation Fractional Distillation Pure Products

Fractional Distillation Protocol

Distillation Definition Distillation Is An Unit Operation Which

Experiment 4 Fractional Distillation Flashcards Quizlet

Solved Fractional Distillation Is Suitable For Separation Of Miscible Liquids With A Boiling Point Difference Of About 25 K Or Less What Part Of Fractional Distillation Apparatus Makes It Efficient And Possess

Difference Between Fractional And Simple Distillation Compare The Difference Between Similar Terms
Difference Between Fractional And Simple Distillation Difference Between
Difference Between Fractional Distillation And Simple Distillation Process Apparatus Uses
Fractional Distillation Is Suitable For Separation Of Miscible Liquids With A Boiling Point Difference Of About 25 K Or Less Studyrankersonline
Purification Fractional Distillation
7 6 Classifying Separation Techniques Chemistry Libretexts
Fractional Distillation Protocol
Fractional Distillation Is Suitable For Separation Of Miscible Liquids With A Boiling Point Difference Of About 25 K Or Less Cbse Class 9 Science Learn Cbse Forum
Difference Between Fractional Distillation And Simple Distillation Process Apparatus Uses
Difference Between Fractional Distillation And Simple Distillation Process Apparatus Uses
Comments
Post a Comment