Beautiful! Why Derivatives Of Phenol Do Not Cause Burns
Sulfuric acid is flushed with a mild soapy solution if the burns are not severe. And dont put butter on burning wounds.
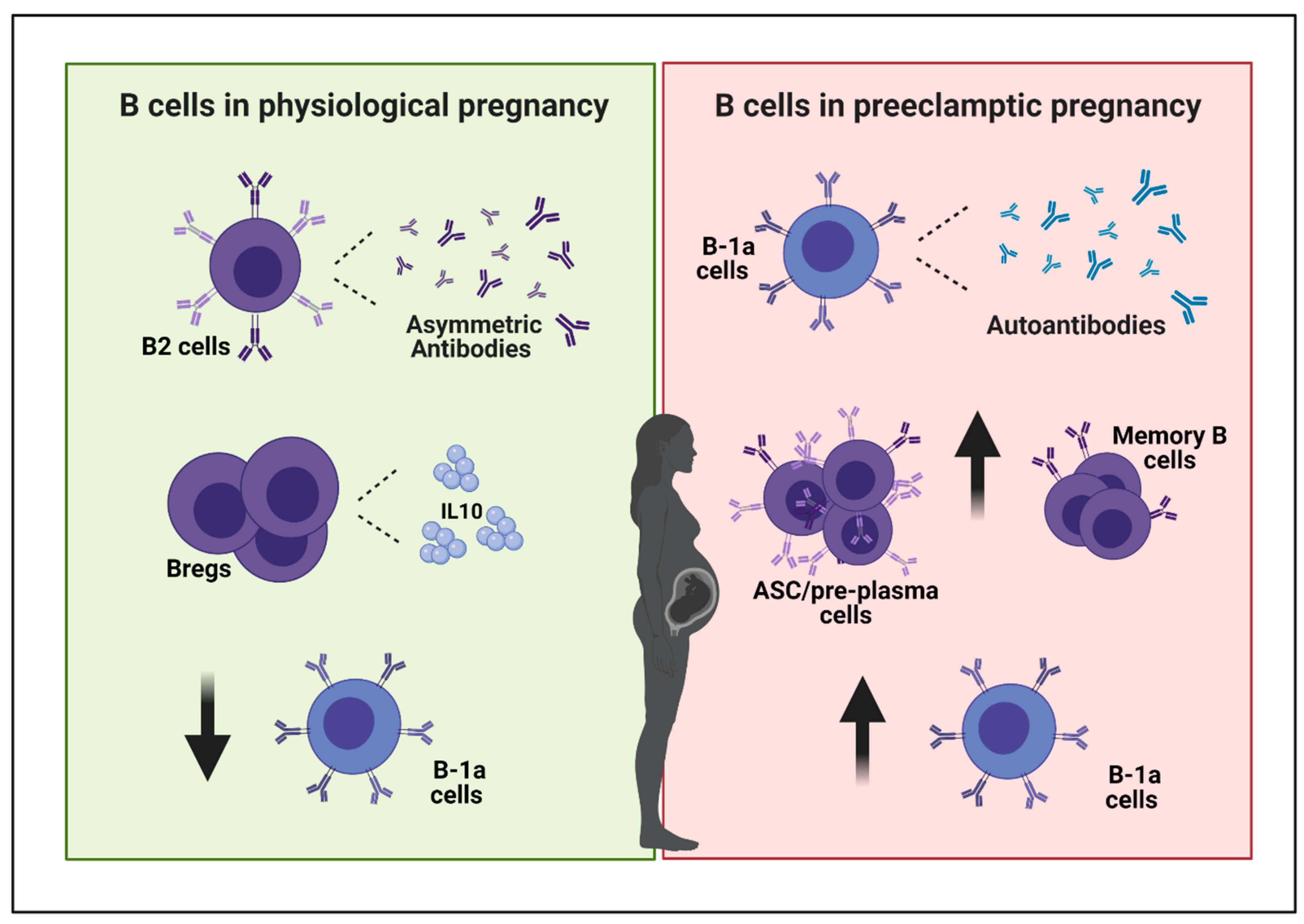
Ijms Free Full Text The Role Of B Cells In Pe Pathophysiology A Potential Target For Perinatal Cell Based Therapy Html
This is a case report of acute severe phenol dermal burn after accidental splash of 94 phenol on 35-year-old patients body who was brought to.
Why derivatives of phenol do not cause burns. Contact with the skin causes a white wrinkled discolouration followed by a severe burn or systemic poisoning if not promptly and properly removed. If phenol is splashed into eyes copious irrigation with water for at least. Cover phenol burns lightly with a clean white cloth.
When used in small controlled quantities and directly applied to the eardrum however phenol can produce safe effective and rapid. Phenol does not build up in fish other animals or plants. The molecule consists of a phenyl -C 6 H 5 group bonded to a hydroxyl -OH group.
It is a mildly acidic water-soluble chemical that requires safe handling due its ability to cause chemical burns. 46 Burns 1994 Vol. Do not use greases powders or ointments in the first-aid treatment of such burns.
He suggested that all the equipment used in the hospitals for surgeries should be sterilized by the disinfectant as they may h. Phenol generally remains in the soil only about 2 to 5 days. Therefore when used in large uncontrolled quantities phenol can cause significant chemical otitis externa.
Phenol is quite toxic however and concentrated solutions cause severe but painless burns of the skin and mucous membranes. It used to be made from Benzene using sulfuric acid and sodium hydroxide in a multi-stage process. High concentrations may cause irritation burns and discolouration to skin mouth throat eyes and airways as phenol is an anaesthetic burns may not be noticed straight away even if they are very serious phenol can be absorbed by the body this may result in nausea vomiting diarrhoea a.
Phenol is the most basic family member of a group of compounds involving an OH group attached to a benzene ring. Phenols are hydroxy aromatic compounds where one or more hydroxyl group are directly attached to the carbon atoms of the benzene ring Phenol is an organic compound which has a great industrial importance It is used as a starting material for the synthesis of many aromatic compounds such as polymers dyes disinfectants salicylic acid derivatives as aspirin picric acid. Short-term exposure to phenol in the air can cause Larger or repeated releases of phenol can remain in the air water and soil for much longer periods of time.
With a strong acid or a strong base the first aid rule is always Water de rest komt later. 9432 Peripheral nervous system No data available. Phenol is a physiological metabolism product.
Phenol burns can result in multiple organ failure. 2007 Indian. Phenols have been used for a long period of time as a disinfectant.
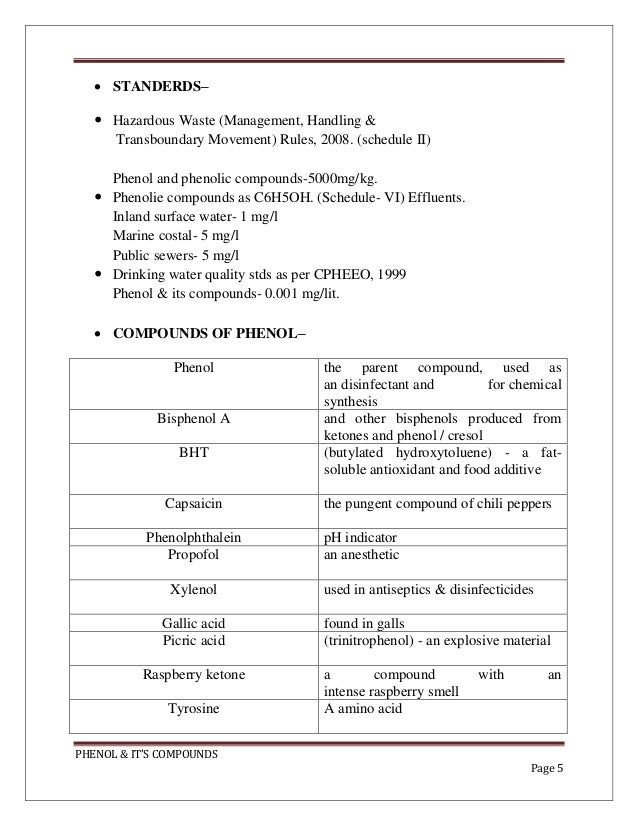
Corrosion inhibition of N80 stee l in h ydrochloric acid by phenol derivatives Vishwanatham S Haldar N. Phenol can remain in water for a week or more. Phenol chemically known as carbolic acid is a white volatile crystal solid.
9434 Skeletal and smooth muscle Locomotor activity reduced at 244 mg phenolkg body weight in female. Phenol 108-95-2 Hazard Summary Exposure to phenol may occur from the use of some medicinal products including throat lozenges and ointments. It has the molecular formula C 6 H 5 OH and is mildly acidic.
Phenol is considered to be quite toxic to humans via oral exposure. Phenol also known as carbolic acid and phenic acid is an organic compound with the formula C 6 H 5 OH. Case a 32 year old male who spilled a solution of phenol over his scalp face neck shoulders and back died 10 minutes later.
Phenol is highly irritating to the skin eyes and mucous membranes in humans after acute short-term inhalation or dermal exposures. A-d Twenty-four-year-old patient 2 h after exposure to a phenol-water solution which splashed over his face trunk arms and legs with a total burned surface area of 205 per cent. Now it is mainly manufactured from benzene and propene with a reaction yield of 86 phenol.
Therefore common phenol was widely used as a disinfectant for surgical instruments which greatly reduced the number of deaths from hospital infection at that time. It is produced on a large scale about 7 billion kgyear. Water the rest comes later.
Phenol may also cause demyelination and axonal damage of peripheral nerves WHO 1994. Less-toxic phenols such as n -hexylresorcinol have supplanted phenol itself in cough drops and other antiseptic applications. The first practical approach of using phenols as disinfectants was suggested by Joseph Lister.
Hospital treatment may include sedation removal of dead tissue fluid therapy and the administration of antibiotics and vitamins. Phenol is produced both naturally as well as synthetically. Phenol creates a small chemical burn at the point where it is directly applied to the TM.
9433 Autonomic nervous system A decrease in body temperature has been reported WHO 1994. Some chemical burns are made worse if rinsed flushed with waterCarbolic acid or phenol does not mix with water so use isopropyl rubbing alcohol first to flush the chemical off the skin and then flush with water. But Phenol is very very nasty you may be able to remove what.
Journal of Chemical Technology 14 5 pp501-506. However over time it has been replaced by its derivatives because phenol is toxic and corrosive and can cause burns. It is a white crystalline solid at room temperature that solidifies at 41C 106F.
Thanks good question.
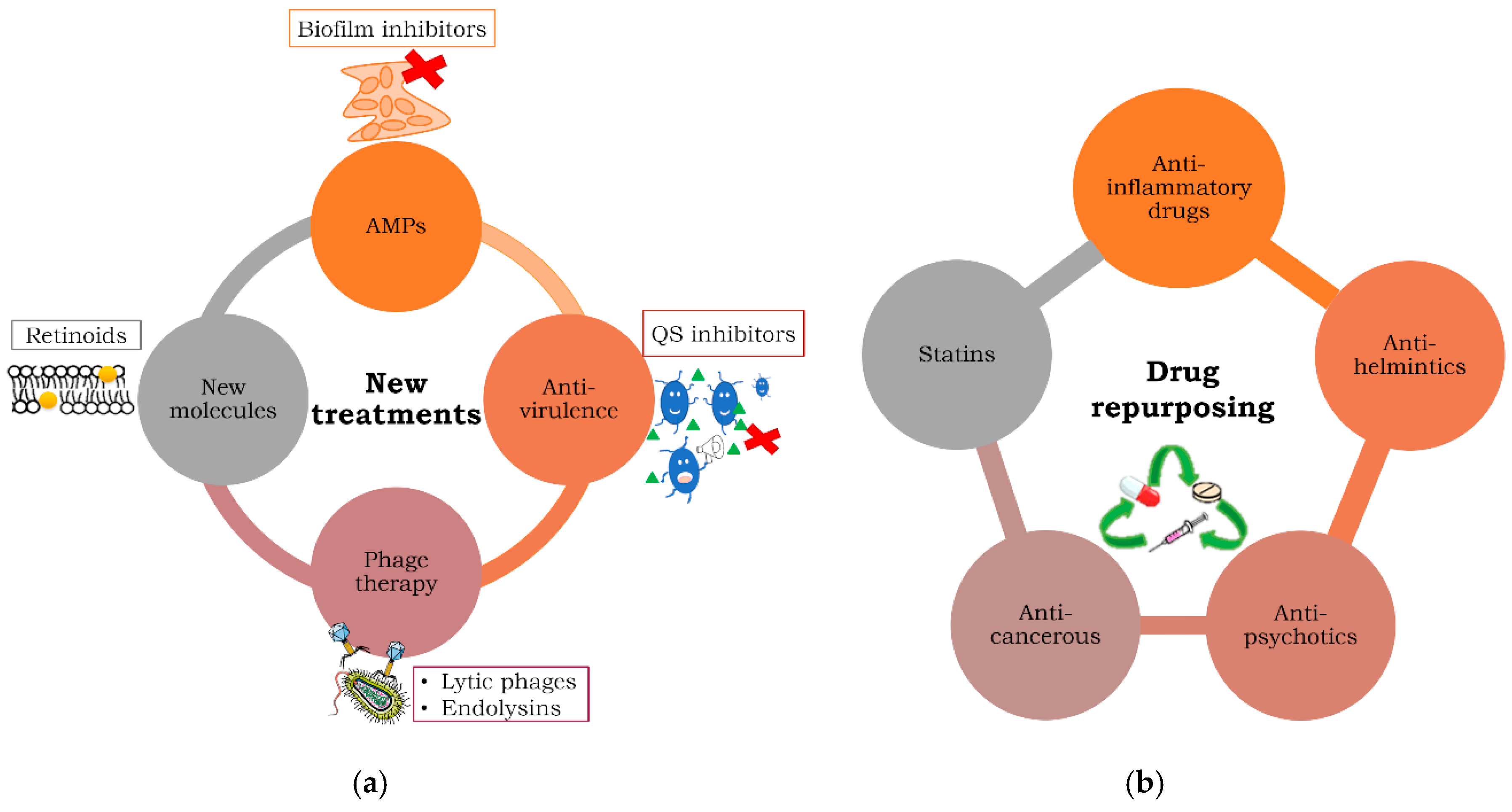
Antibiotics Free Full Text Strategies To Combat Multidrug Resistant And Persistent Infectious Diseases Html
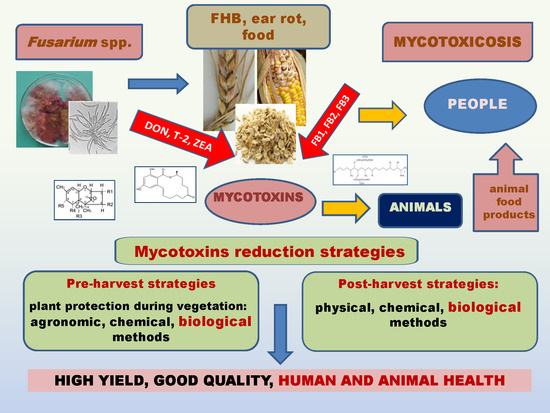
Agronomy Free Full Text Fusarium Head Blight Mycotoxins And Strategies For Their Reduction Html

A Brief Summary Of Disinfectants Antiseptics Compound Interest

Quinoline Is A Heterocyclic Aromatic Organic Compound With The Chemical Formula C9h7n It Is Cooking Steak On Grill Cooking Oatmeal Cooking Light Recipes

Pyridine Is A Basic Heterocyclic Organic Compound With The Chemical Formula C5h5n It Is Structurally Related To Benzene Pyridine Chemistry Chemistry Art

Tbbq Does Not Cause Visible Damage To A Living Skin Equivalent Labskin Download Scientific Diagram
Dentistry Journal Free Full Text Oral White Lesions An Updated Clinical Diagnostic Decision Tree Html

Pin By Caitlin Magarger On Physics Education Teaching Chemistry Biology College Chemistry Education

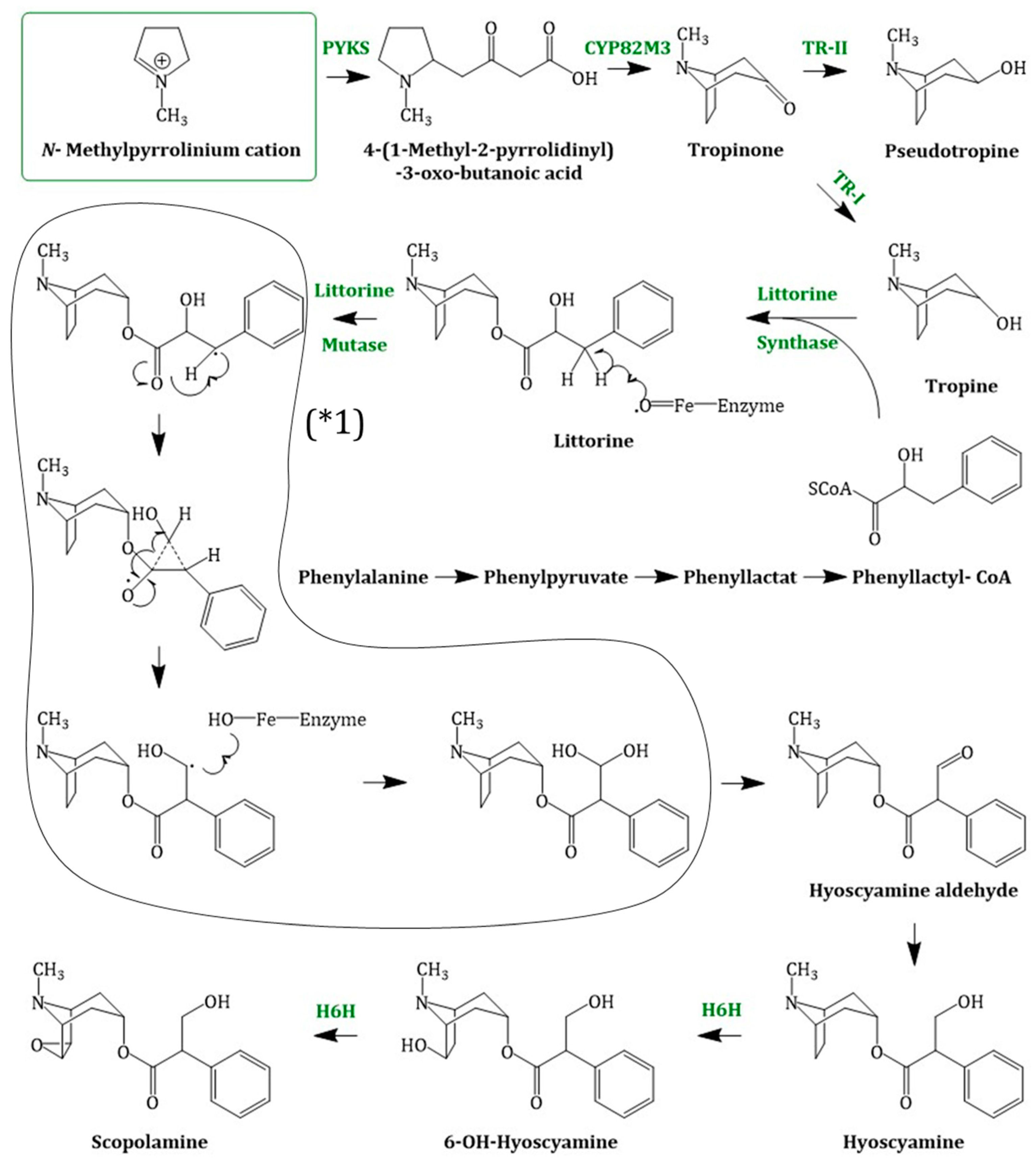
Molecules Free Full Text Tropane Alkaloids Chemistry Pharmacology Biosynthesis And Production Html

Polymers Free Full Text Water Soluble Photoinitiators In Biomedical Applications Html

Propyleneglycol Iupac Name Propane 12 Diol Is A Synthetic Organic Compound With The Chemical Formula C3h8o2 It Is A Viscous Colorless Liquid Which Is Ne

Tbbq Does Not Cause Visible Damage To A Living Skin Equivalent Labskin Download Scientific Diagram

Molecules Free Full Text Acai Euterpe Oleracea Mart Seed Extract Induces Ros Production And Cell Death In Mcf 7 Breast Cancer Cell Line Html




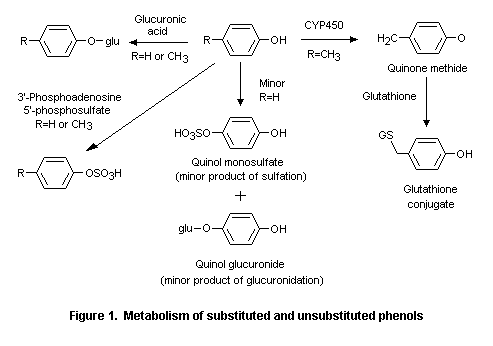
Comments
Post a Comment