OH MY GOD! Explain Why Glass Is Described As Hydrophilic
At hydrophilic surfaces the complex three-dimensional network of water molecules collapses and creates a water layer inbetween with higher density than that of bulk water leading to repulsive interactions between hydrophilic surfaces In contrast at hydrophobic surfaces the water molecule network is stretched leading to a water layer with. What properties of water are at work.
Materials Free Full Text A Review On Development And Applications Of Bio Inspired Superhydrophobic Textiles Html
Water move through plants 1.

Explain why glass is described as hydrophilic. In the back of the book Science Instruction in the Middle and Secondary Schools 2002 the authors Eugene Chiapetta and Thomas Koballa suggest that if you place a drop of water on a piece of wax paper and ask the question Does the drop of water roll or slide across the paper. From its humble beginnings as a window pane in luxury houses of Pompeii to sophisticated structural. Hydrogen bonds link the glass and water molecules by adhesion 3.
-the hydrogen bonds between H2O molecules allows for temporary interaction that lead to its fluid nature. The oil water and soap is the best that at maintaining the emulsion. The amino group and carboxyl group make it pretty hydrophilic as illustrated by the fact that its water solubility is over 150 gL.
Glass is different perfectly clean glass will allow water to adhere to. Adding hydrophobic rain-repellent glass treatments to windshields causes water to bead and roll off the windshield surface to improve visibility and safety. Why is glass described as hydrophilic.
Buffers pH Acids and Bases. The partial charges on a water molecule occur because of _____. 3 water is one of the many hydrophobic molecules.
But when we categorize amino acids we are generally talking about their side chains. 4 the atoms in water have equal electronegativities. Free alanine isnt really.
List the properties of water that make it a valuable solvent. Water rises up the tube due to water molecules linked together from cohesion furthermore these water molecules also attached to the surface of the tube from adhesion allowing. At first thought to possess magical properties glass has come a long way.
Describe challenges faced when designing and depositing thin surface films Explain why companies would require hydrophobic surfaces on materials Describe the ideal properties of a. Explain why glass is described as hydrophilic. The formation of a conditioning film on a surface has a profound influence on its chemistry and therewith on the above physico-chemical properties.
Hydrophilic molecules are polar molecules. The term hydro-Philic means water loving compared to hydro-Phobic or water fearing. The potential reason could be hydrophilic acrylic IOLs are machined in the dehydrated state and then rehydrated which can lead to loss of edge sharpness.
Distinguish between hydrophilic and hydrophobic substances. Bacterial adhesion to CL surfaces is determined by the surface properties of the bacterial cell the lens and the suspending liquid like hydrophobicity or surface free energy and electrostatic charge or zeta potential. An emulsion is defined to be a mixture where two immiscible fluids where one liquid is dispersed as in by droplets into the other.
1 the opposite ends of the molecule have opposite electrical charges. Place a glass slide on a good size area of paper towels old newspaper or. Hydrophilic molecules are molecules that can dissolve in water.
These differences in manufacturing techniques may explain why hydrophobic IOLs appear to have relatively better PCO performance. The pH of a solution is a measure of its acidity or alkalinity. -water is a polar covalent bond that are attracted to other polar covalent molecules.
It is one of the most versatile and oldest materials in the building industry. The hydrophilic character of a molecule can be described as its hydrophilicity. Nonpolar molecules that repel the water molecules are said to be hydrophobic.
You have probably used litmus paper paper that has been treated with a natural water-soluble dye so it can be used as a pH indicator to test how much acid or base alkalinity exists in a solutionYou might have even used some to make sure the water in an outdoor swimming pool is properly. This property of water was important for the evolution of life. That would explain why superimposed ice was enriched in the less expected hydrophobic compounds rather than the hydrophilic ones which should be more abundant in meltwater that feeds the SI layer.
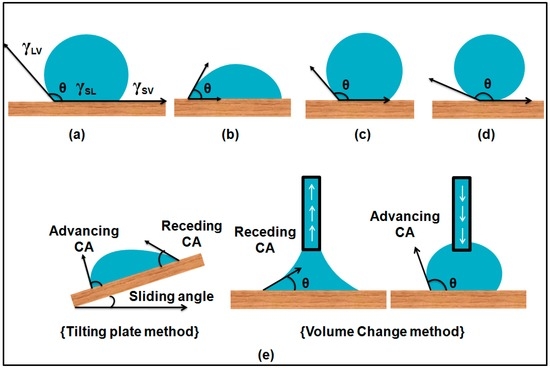
Explain why on a molecular level. Answer 1 of 2. This is called the contact angle.
This would suggest a similar source of chemicals for both types of layers perhaps even resulting from a water surface mechanism. Self-cleaning glass is a specific type of glass with a surface that keeps itself free of dirt and grime. 2 water molecules are linear like a pole.
Most waxes and plastics are hydrophobic and water just runs off the surface. Adhesion water molecules sticking to glass 2. Applying a hydrophilic anti-fog coating to glass causes any condensation to form into a thin even layer of water instead of droplets so the glass remains transparent.
Water molecules are polar molecules which allows polar molecules to get dissolved in water. According to James Stevens Curl Susan Wilson Authors of The Oxford Dictionary of architecture glass is a semi- or fully transparent hard brittle lustrous material made by igneous fusion of silica usually sand with an alkaline sodium or potassium salt and added ingredients. You will have a puzzle that needs to be resolved and explained.
If the droplet spreads wetting a large area of the surface then the contact angle is less than 90 degrees and that surface is. Glass has been a fascinating material to humankind since it was first made in about 500 BC. Hydrophilic and hydrophobic materials are defined by the geometry of water on a flat surface specifically the angle between a droplets edge and the surface underneath it.
The field of self-cleaning coatings on glass is divided into two categories. Molecules forming ionic or a hydrogen bond with the water molecule are said to be hydrophilic. It appears to have come into use for glazing the windows of grander.
That is the hydrophilic molecules attract water molecules. Hydrophobic and hydrophilicThese two types of coating both clean themselves through the action of water the former by rolling droplets and the latter by sheeting water that carries away dirt. 5 all of the above.
Water sticks to the glass instead of repelling it as oil would do.

Solved Nas Www Heron Exercise 4 Lab Report Properties Of Chegg Com

Explained Hydrophobic And Hydrophilic Mit News Massachusetts Institute Of Technology
What Are Some Examples Of Hydrophilic Substances Quora

Fabrication Of Hydrogel Particles Of Defined Shapes Using Superhydrophobic Hydrophilic Micropatterns Neto 2016 Advanced Materials Wiley Online Library
Solved 1 1 Pt Is Being Hydrophobic Hydrophilic A Chegg Com

Hydrophilic Glass An Overview Sciencedirect Topics
Defined Hydrophilic Hydrophobic Oleophilic Oleophobic Hygroscopic

Hydrophilic Glass An Overview Sciencedirect Topics
Solved 1 When You Blow Bubbles Into A Glass Of Water The Chegg Com

Hydrophilic Glass An Overview Sciencedirect Topics

Hydrophilic Glass An Overview Sciencedirect Topics

What Is Hydrophobic Glass Affordable Auto Glass In Minnesota

Hydrophilic Glass An Overview Sciencedirect Topics

Phospholipids Biology For Majors I
Are Polar Molecules Considered Hydrophilic Why Or Why Not Quora
Difference Between Hydrophilic And Hydrophobic Difference Between

Hydrophilic Glass An Overview Sciencedirect Topics

Comments
Post a Comment